PROEFSCHRIFT
Quantification of P-glycoprotein function using [18F]MC225 PET
- 24-09-2025
Over the past few decades, immuno-PET has demonstrated its value by enabling both visual and quantitative investigations of the immune system in vivo (1). In the development of monoclonal antibody (mAb)-based therapy, immuno-PET helps answering important questions related to 1) the pharmacokinetics of the drug or molecule: “What is the distribution of the drug or molecule?”; 2) study optimisation: “What is the optimal imaging mass dose and time point?”; and 3) target engagement: “Does the drug or molecule reach and interact with the target?”. Immunohistochemistry (IHC) is traditionally used to assess protein expression in tumours before applying mAb-based treatments targeting that protein. However, the reliability of IHC may be affected by intra- and intertumoural heterogeneity and dynamic changes in target expression due to therapy or target recycling. Immuno-PET imaging provides advantages over IHC regarding these concerns. The focus of this PhD project was on assessing optimal quantification methods for the immuno-PET tracers [18F]F-AraG and 89Zr-mAbs, giving insight into tracer pharmacokinetics, target engagement and study optimisation.
[18F]F-AraG PET imaging
Recently, [18F]F-AraG was developed as a novel PET tracer for imaging activated T cells (2). An important step in the development of a PET tracer is characterising its pharmacokinetics to validate simplified uptake measures for the quantification of PET uptake. The pharmacokinetics of [18F]F-AraG tumour lesion uptake were analysed in thirteen patients with non-small cell lung cancer (NSCLC). The dynamic blood sampling and PET imaging best fit an irreversible model with the Ki as a measure for irreversible uptake. The irreversible kinetics of [18F]F-AraG in tumour tissue correspond to the proposed biological mechanism of [18F]F-AraG uptake in the T cell. Blood-corrected uptake measures, tumour-to-blood ratio (TBR) and tumour-to-plasma ratio (TPR) showed the strongest correlations with Ki, thus demonstrating the greatest potential for accurately quantifying tumour lesion [18F]F-AraG uptake in patients with NSCLC.
Quantification of 89Zr-immuno-PET
In contrast to 18F-labelled tracers, 89Zr-mAbs exhibit different distribution characteristics due to their relatively large size. In conventional pharmacokinetic modelling, tracer uptake is quantified dynamically during the distribution and uptake time of the tracer, as applied to [18F]F-AraG. Due to their slow distribution, 89Zr-mAbs would require a PET scan with a duration of several days, which is impossible. Instead, multiple static PET scans are obtained on the days after tracer administration to provide dynamic information. Additionally, an irreversible model is assumed based on the residualizing characteristic of 89Zr leading to the accumulation of 89Zr within the tissue after target binding (1). This allows the use of Patlak graphical analysis (3), in which the data from multiple static PET scans are combined into one analysis. This analysis can be regarded as a simplified approach to obtain the Ki value, representing the rate of irreversible uptake. The effect of different imaging time points of 89Zr-immuno-PET scans on the accuracy and precision of Patlak results was investigated during a simulation study. Inclusion of a blood sample and PET scan 24 hours after tracer administration provided more accurate and precise results as compared to without, while the exact timing of the other two time points was not critical.
It is desirable to use simplified uptake measures obtained from a single PET scan to quantify 89Zr-mAb uptake. While the standardised uptake value (SUV) is commonly used in PET imaging to quantify uptake, blood-corrected uptake methods such as TBR or TPR may be more appropriate for 89Zr-immuno-PET because of the distribution of mAbs. Simplified uptake measures, SUVBW, TPR, and TBR, were validated against the Patlak-derived Ki value for quantification of 89Zr-mAb uptake in tumours. When patients received similar mass doses, the use of SUVBW was valid for quantifying irreversible uptake and comparing between patients. However, when variable mass doses of unlabelled mAbs were administered, a TBR or TPR was shown to be more reliable for quantifying irreversible uptake compared to SUVBW. A TBR or TPR accounts for mass-dose-specific plasma activity concentrations (or plasma kinetics), which SUVBW does not consider.
89Zr-Immuno-PET as a tool to assess target engagement
Furthermore, understanding the pharmacokinetics underlying the quantification of 89Zr-immuno-PET is essential for distinguishing actual target-specific uptake from the total measured PET signal, which also includes non-target-specific uptake (see figure). Previous literature provided two approaches to separate target-specific from non-target-specific uptake. The first is assessing whether the measured irreversible uptake exceeds a baseline Patlak-derived Ki value that represents non-target-specific irreversible uptake in that organ (4). The second is assessing whether irreversible uptake decreases with increasing mass doses, as this saturation effect indicates target engagement and therefore suggests target expression (5).
Both these approaches were applied to PET imaging to demonstrate target engagement of immune checkpoint-targeting 89Zr-mAbs in healthy organs. Overall, low mass doses resulted in uptake above baseline Ki values, and the uptake decreased with higher mass doses, both indicative of target engagement of the specific 89Zr-mAb in the assessed organ.
Additionally, the presence of non-target-specific irreversible 89Zr-mAb uptake in tumours was investigated. To assess non-target-specific irreversible uptake, Patlak graphical analysis was applied to data from two 89Zr-immuno-PET studies that included incidental findings of biopsy-proven target-negative tumours. Irreversible 89Zr-mAb uptake in target-negative tumours was lower than in target-positive tumours and above zero, suggesting non-target-specific irreversible uptake in tumours. Although the sample size was small, the study highlighted the importance of accounting for non-target-specific irreversible uptake of 89Zr-mAbs in tumours when quantifying and interpreting tumour uptake.
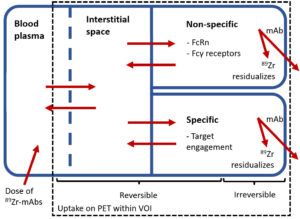
Figuur 1. Schematic representation of the distribution and elimination of 89Zr-mAbs within the body. 89Zr-mAbs are administered to the blood plasma, distribute and are reversibly present inside the blood fraction and interstitial space of the tissue. Subsequently, several specific (binding to the target receptor) and non-specific (e.g. binding to FcRn within endothelial cells and Fcγ receptors on immunological cells) binding processes occur, which could both be reversible and irreversible. After irreversible binding of 89Zr-mAb, 89Zr residualizes inside the cell leading to accumulation of PET signal within the volume of interest (VOI). 89Zr-mAbs = zirconium-89 labelled monoclonal antibodies; FcRn = neonatal Fc-receptor; VOI = volume of interest
Conclusion
As immuno-PET demonstrates significant potential in the development of mAb-based therapy, valid quantification is crucial for interpreting immuno-PET scans. The quantification of immuno-PET advances towards actual measurements of target expression, unlocking new possibilities in drug development.
The full thesis is published online: https://research.vu.nl/en/publications/quantification-of-immuno-pet-imaging-in-cancer
References
- Van Dongen G, Huisman M, Boellaard R, et al. 89Zr-immuno-PET for imaging of long circulating drugs and disease targets: why, how and when to be applied? Quarterly Journal of Nuclear Medicine and Molecular Imaging. 2015;59:18-38
- 2. Namavari M, Chang YF, Kusler B, et al. Synthesis of 2′-deoxy-2′-[18F]fluoro-9-beta-D-arabinofuranosylguanine: a novel agent for imaging T-cell activation with PET. Mol Imaging Biol. 2011;13:812-8. doi:10.1007/s11307-010-0414-x
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Journal of Cerebral Blood Flow & Metabolism. 1983;3:1-7
- Jauw YWS, O’Donoghue JA, Zijlstra JM, et al. (89)Zr-Immuno-PET: Toward a Noninvasive Clinical Tool to Measure Target Engagement of Therapeutic Antibodies In Vivo. J Nucl Med. 2019;60:1825-32
- 5. Menke-van der Houven van Oordt CW, McGeoch A, Bergstrom M, et al. Immuno-PET Imaging to Assess Target Engagement: Experience from (89)Zr-Anti-HER3 mAb (GSK2849330) in Patients with Solid Tumors. J Nucl Med. 2019;60:902-9. doi:10.2967/jnumed.118.214726
Jessica Wijngaarden, PhD

Jessica Wijngaarden, PhD
19 maart 2025
Vrije Universiteit Amsterdam
Promotores:
Prof. Ronald Boellaard, PhD
Prof. C. Willemien Menke-van der Houven van Oordt, MD, PhD
Co-promotor:
Ir. Marc C. Huisman, PhD