PROEFSCHRIFT
Quantification of P-glycoprotein function using [18F]MC225 PET
- 24-09-2025
P-glycoprotein (P-gp), a member of the ATP-binding cassette (ABC) transporter family, plays a vital role in drug distribution, barrier function, and disease mechanisms. It is highly expressed at biological interfaces such as the blood-brain barrier (BBB), gastrointestinal tract, liver and kidneys, where it actively effluxes xenobiotics and therapeutic agents, thereby affecting drug efficacy and resistance. Beyond drug delivery, P-gp is involved in neurodegenerative diseases, epilepsy and cancer resistance, making it a key target in clinical research. In the central nervous system (CNS), P-gp extrudes substrates to protect neural integrity and prevent the accumulation of harmful compounds. Dysfunctional P-gp has been linked to disorders such as Parkinson’s disease (PD), where impaired efflux may cause the accumulation of neurotoxic proteins, such as α-synuclein. This dysfunction, together with BBB disruption, may accelerate disease progression by allowing toxic substances to accumulate in the brain.
Given its critical role in maintaining CNS homeostasis, understanding the factors that regulate P-gp function is essential. Genetic polymorphisms, pathologies, and environmental factors have an effect on P-gp function, affecting pharmacokinetics and drug efficacy. Positron emission tomography (PET) using positron emitting tracers, such as [18F]MC225, facilitates non-invasive in vivo assessment of P-gp function.
The work presented in the thesis aimed to refine P-gp quantification methods for [18F]MC225 PET, investigating sex-related differences, neurodegenerative disorders and alternative tracer administration routes for assessing the gut-brain axis. Five studies were designed to address these objectives.
The first study investigated a method for generating blood time-activity curves (BTACs) without the need for continuous arterial sampling. Aortic arch-derived BTAC (BTACAA) showed discrepancies compared with manual arterial samples, underestimating at early and overestimating at late time points. When calibrated using manual arterial samples, however, BTACAA_CAL provided reliable estimates of volume of distribution (VT). Similarly, internal carotid artery (ICA)-derived BTAC, calibrated with manual arterial samples, yielded consistent VT results comparable with BTACAA_CAL, highlighting its potential as an alternative input function source for PET quantification. Meanwhile, venous sampling reliably estimated plasma-to-whole blood ratios and plasma parent fractions, but failed to calibrate BTAC and estimate VT.
Another objective was to explore potential sex-related differences in P-gp function at the BBB in a cohort of healthy older adults (55+) using [18F]MC225 PET imaging. Results of this study showed that male and female participants exhibited no substantial differences in VT values across various brain regions. These findings suggest that sex does not appear to affect P-gp function at the BBB in this age group. However, further research in younger populations may be needed to investigate the possible effects of hormonal differences on P-gp function.
To investigate the clinical relevance of P-gp function in neurodegenerative disorders, the thesis also examined P-gp function in the brain and small intestines of four patients with Parkinson’s disease (PD) (figure 1). No significant differences in VT values were observed between brain and small intestines of PD patients and healthy controls. However, regional variations in brain VT and a trend towards lower intestinal VT in PD warrant further investigations in larger cohorts to explore the effects of disease stage and symptom severity.
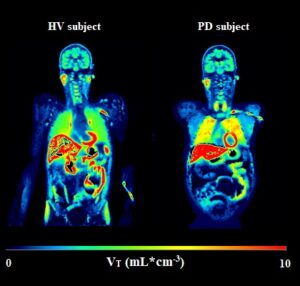
Figure 1. Parametric images showing the volume of distribution (VT) values in a representative healthy volunteer (HV) and a Parkinson’s disease (PD) patient.
Expanding on the potential of alternative tracer administration routes, a scoping review was conducted to evaluate the use of oral administration in PET imaging. It identified studies involving oral administration in humans, primates, and rodents. Unfortunately, there was substantial variability across studies in acquisition protocols, quantification methods and pharmacokinetic measures. Despite this, oral tracer administration could be a valuable tool for quantitatively characterising the gastrointestinal tract and understanding the absorption, distribution, metabolism and excretion of various compounds, including vitamins, pharmaceuticals, plastics and peptoids. In addition, this route offers insights into the dynamics of the gut-brain axis, enabling the quantitative study of how compounds transit and interact within the gastrointestinal tract and reach the brain.
Finally, a preclinical study investigated the feasibility of administering [18F]MC225 orally. This study demonstrated that fasting and modified gavage techniques improved concentration levels and visualisation of the brain. However, in contrast to expectations, the P-gp inhibitor tariquidar did not increase brain tracer concentrations, indicating that the observed signals were mostly due to non-specific uptake. Figure 2 illustrates both brain and abdominal PET images for each group investigated in this study. Stability studies confirmed that [18F]MC225 remains stable under simulated gastric conditions, implying possible intestinal metabolism involving P-gp and CYP3A4.
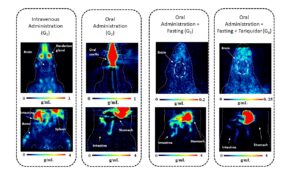
Figure 2. Coronal slices through brain images for each group investigated are presented in standardised uptake values (SUV in g/mL). Static images were acquired from 0 to 90 minutes for the intravenous administration group (G1) and the oral administration group (G2), and from 0 to 96 minutes for the oral administration plus fasting group (G3) and the oral administration plus fasting and tariquidar group (G4). In addition, 15 min static [18F]MC225 PET images of the abdomen are depicted. Abdominal scans are not directly comparable between groups due to differences in acquisition time: for G1 and G2, scans were acquired 98 minutes after tracer administration, while for G3 and G4, scans were acquired 248 minutes after tracer administration.
In conclusion, this thesis furthers the understanding of [18F]MC225 PET imaging and its potential to elucidate the role of P-gp in health and disease. The work identifies methodological limitations, such as partial volume effects and motion correction for intestinal imaging, and proposes future directions, including expanding clinical PD studies and non-invasive quantification methods, as well as investigations into the role of environmental factors, food intake, and microbiota in modulating P-gp function.
The complete thesis can be found online at: 10.33612/diss.1279090531
Giordana Salvi de Souza, PhD

Giordana Salvi de Souza, PhD
23rd of April 2025
University of Groningen
Promotores:
Prof. C. Tsoumpas, PhD
Prof. A. A. Lammertsma, PhD
Co-promotores
Luurtsema, PhD
R. G. Furini, PhD